Remote residue participation in Enzyme catalysis
We study the effect of remote residues in enzyme catalysis using computational predictions and experimental verification by site-directed mutagenesis, kinetics assays, and ligand binding studies. The big news is that the participation of remote residues in enzyme catalysis is predictable with a simple calculation.
See:
Probing remote residues important for catalysis in Escherichia coli ornithine transcarbamoylase
Lisa Ngu, Jenifer N. Winters, Kien Nguyen, Kevin E. Ramos, Nicholas A. DeLateur, Lee Makowski, Paul C. Whitford, Mary Jo Ondrechen, Penny J. Beuning, PLoS ONE 15(2): e0228487 (2020)
See:
Prediction of distal residue participation in enzyme catalysis
Heather R. Brodkin, Nicholas A. DeLateur, Srinivas Somarowthu, Caitlyn L. Mills, Walter R. Novak, Penny J. Beuning, Dagmar Ringe, Mary Jo Ondrechen, Protein Science 24 (5), 762-778 (2015)
Here are some examples:
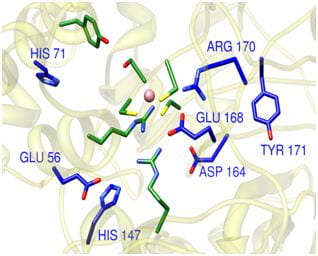
Figure 1
Variants made of the remote residues predicted by THEMATICS in the enzyme Nitrile Hydratase show significant decrease in catalytic efficiency.
See Heather R. Brodkin, W.R.P. Novak, et al. Biochemistry 50(22), 4923-4935 (2011)
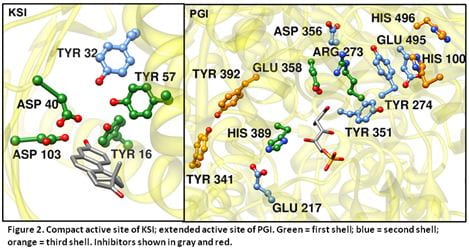
Figure 2
THEMATICS predicts a single-layered active site for Ketosteroid isomerase and a multilayered active site for PGI. Site-directed mutagenesis and kinetics assays confirm these predictions.
See Srinivas Somarowthu, Heather R. Brodkin, J.A. D’Aquino, Dagmar Ringe, Mary Jo Ondrechen, and Penny J. Beuning, Biochemistry 50(43), 9283-9295 (2011)
This work is funded by the National Science Foundation via grant # MCB-1158176 and MCB-1517290.